Difference between revisions of "Struvite recovery"
| Line 53: | Line 53: | ||
====Manufacturing==== | ====Manufacturing==== | ||
| − | [[Image:Struvite reactor.jpg|thumb|right|150px|Diagram of a | + | [[Image:Struvite reactor.jpg|thumb|right|150px|Diagram of a STUN reactor]] |
For the production of struvite it is necessary to manufacture a struvite harvesting reactor. This can be of different sizes depending on the scale of struvite production. The reactor and materials used can be adapted depending on the funds and materials available. | For the production of struvite it is necessary to manufacture a struvite harvesting reactor. This can be of different sizes depending on the scale of struvite production. The reactor and materials used can be adapted depending on the funds and materials available. | ||
Revision as of 11:18, 7 September 2010
Urine is often used as a liquid fertilizer in rural areas due to its highly concentrated nutrient content. The main minerals required by plants are nitrogen, potassium and phosphorus. Urine contains roughly 80% of the nitrogen, 60% of the potassium and 55% of the phosphorus that humans excrete. While nitrogen is relatively easy to obtain from other sources, potassium and phosphorus are more scarce. The only readily available organic source of these is urine. However application of urine is often unwanted since it is required in large volumes, is inconvenient to transport and has a bad odor. Also it cannot be applied through irrigation systems.
When urine is stored, a spontaneous reaction occurs, forming the precipitate: magnesium ammonium phosphate also known as struvite. However only 30-50% of the phosphate precipitates naturally. By reacting the urine with magnesium, over 90% of the phosphorus can be recovered. This can be filtered out and dried to produce a valuable powder fertilizer. The remaining effluent contains most of the urine’s nitrogen and potassium. Since there are no precipitates and can be applied to crops through irrigation systems.
Struvite can be produced locally using a locally manufactured reactor. Currently the capital costs of the reactor are quite high and it must be built by skilled workers, so struvite production is not economically viable in all communities. However research is still being done to improve designs of the reactor and develop low cost business models.
| Advantages | Disadvantages/limitations |
|---|---|
| - It is a biological product and does not contain any heavy metals or pharmaceuticals - It has a constant composition, unlike urine, preventing excess minerals poisoning the soil |
- Requires urine to be separated from other sanitation products - Currently not economically viable due to high capital costs |
History and social context
Pilot studies were conducted by Eawag and UN-Habitat in a peri-urban farming community called Siddhipur in the Kathmandu Valley in Nepal. This was done to investigate the feasibility of urine separation and local struvite production.
The urine was separated using the already existing EcoSan toilets in each household. Cyclists went around the neighborhood to collect the urine and transport it to the reactor. 400L of urine was used for struvite production daily.
The study showed that struvite recovery is indeed feasible and a community wide production programme could generate around 170kg of struvite per year.
Suitable conditions
Struvite production is basically suitable in areas where urine application is possible. It requires the implementation of EcoSan systems in the community. It might have to be used in combination with other fertilizers, such as the effluent left when struvite is precipitated. This can be used in irrigation systems to directly fertilize crops.
Further for the business model to be sustainable, a local magnesium source is required.
Technical specification
Operation
For the production of struvite, firstly urine is needed. This can be collected from markets, urinals, public buildings. It must be separated from other sanitation products using urine diverting toilets or other EcoSan technologies. Then magnesium must be obtained. This can come from bittern from salt production, which contains 3-10% magnesium, or magnesium sulphate from fertilizer powder, containing 5-10% magnesium.
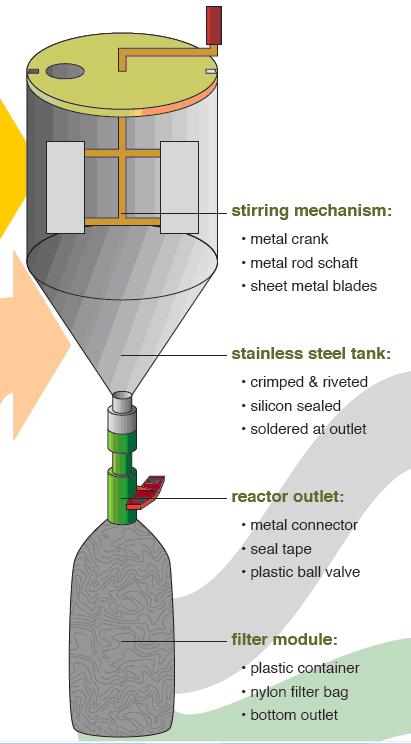
Using the STUN reactor: First the reactor is filled with urine. Then the magnesium is added. The molar ratio of magnesium to phosphorus in the urine should be 1:1. Hence the phosphorus concentration needs to be determined and depending on the magnesium source, the appropriate amount added. The mixture stirred manually by rotating the crank handle. This is done for about 10 minutes as the struvite precipitates. After the struvite has settled, the valve is opened to filter out the struvite. This is then sundried. The leftover effluent can be used for irrigation. It is suitable for drip irrigation systems.
The reactor has a treatment capacity of 10L per litre volume of the reactor per day. Reactors can be built up to volumes of 500L, which then has a capacity of 5000L per day.
Maintenance
Manufacturing
For the production of struvite it is necessary to manufacture a struvite harvesting reactor. This can be of different sizes depending on the scale of struvite production. The reactor and materials used can be adapted depending on the funds and materials available.
The STUN reactor, consists of a stainless steel tank, a stirring mechanism, a reactor outlet and filter module. Usually, a tank of stainless steel sheet with a conical bottom is assembled. It is sealed with silicon and soldered at the outlet. Then the stirring mechanism and stand are constructed by welding together metal bars and sheets. Then a plastic container is connected to the outlet, with a nylon bag inside to filter the water.
Estimated Lifespan
Cost
| Captial Cost | Operation Cost | Replacement Cost | Estimated 5 years Cost | Cost/liter treated |
|---|---|---|---|---|
| US$ 571.95 * | US$ | US$ | US$ | US$ |
- For a 500L reactor in Nepal.
Country experiences
External Links

|