Difference between revisions of "Desalination / Evaporation"
(→Reference manuals, videos, and links) |
(→Acknowledgements) |
||
| Line 40: | Line 40: | ||
==Acknowledgements== | ==Acknowledgements== | ||
* [http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CDAQFjAB&url=http%3A%2F%2Fwhqlibdoc.who.int%2Fpublications%2F2011%2F9789241548151_eng.pdf&ei=RlCHT5-jGeOiiQL5xKmLAg&usg=AFQjCNGx2Q3Rc5yFmbygAIgmJOjg5CSp7g&sig2=C9T9N9V9nUigsjUZtqMR5w ''Guidelines for Drinking-water Quality''], fourth edition. World Health Organization (WHO), 2011. | * [http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CDAQFjAB&url=http%3A%2F%2Fwhqlibdoc.who.int%2Fpublications%2F2011%2F9789241548151_eng.pdf&ei=RlCHT5-jGeOiiQL5xKmLAg&usg=AFQjCNGx2Q3Rc5yFmbygAIgmJOjg5CSp7g&sig2=C9T9N9V9nUigsjUZtqMR5w ''Guidelines for Drinking-water Quality''], fourth edition. World Health Organization (WHO), 2011. | ||
| + | * Smith, Michael, and Shaw, Rod. [http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CDEQFjAA&url=http%3A%2F%2Fwww.lboro.ac.uk%2Fwell%2Fresources%2Ftechnical-briefs%2F40-desalination.pdf&ei=zFuHT-2kIJHYiQLUy4myAg&usg=AFQjCNEhxN_0q4aUgKOuu1ep8fUxBp4Cig&sig2=mdlJUIaqoJdLexf7TVT0Yw Desalination]. WEDC Loughborough University. | ||
Revision as of 23:53, 12 April 2012
Desalination is used to remove salts from brackish or saline surface water and groundwater in order to render it acceptable for human consumption or other uses. Brackish water is defined as starting at having a Total Dissolved Solids content of 1,000 mg/l, and saline water as having 10,000 mg/l. It is increasingly employed to provide drinking-water because of a growing scarcity of fresh water driven by population growth, overexploitation of water resources and climate change. Desalination facilities exist all over the world, particularly in the eastern Mediterranean region, with use increasing on all continents. Small-scale desalination is used to supply fresh water on ships and to provide additional fresh water in some hot and arid regions.
Desalination by distillation produces water without chemical salts. The method can be expensive because of the capital investment needed and because fuel/charcoal is used to heat the water. The volume of water produced is also low.
Most present applications of desalination are for estuarine water, coastal water and seawater. Desalination may also be applied to brackish inland waters (both surface water and groundwater) and may be used on board vessels. Small-scale desalination units also exist for household and community use and present specific challenges to effective operation and maintenance.
Contents
Suitable Conditions
For use in areas where there are few alternative water sources to the available saline water.
Construction, operations and maintenance
Of the desalination methods available, the two main ones are: distillation followed by condensation and reverse osmosis.
Alternative low-cost distillation method
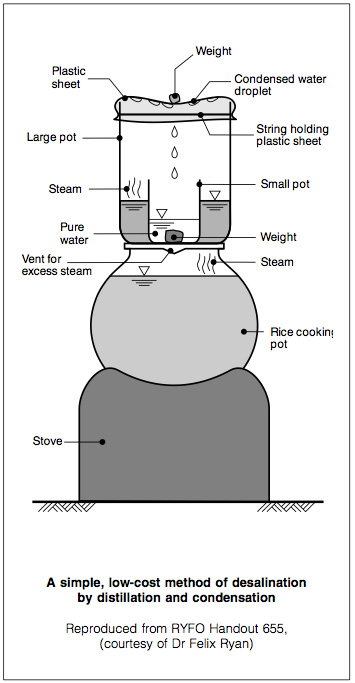
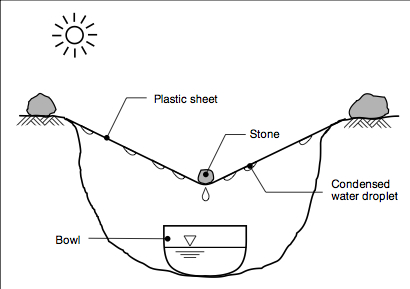
A simple, low-cost method to desalinate sea water by distillation is used in some countries where fuel is available. It requires basic kitchen utensils: two pots, one four times the size of the other, and a plastic sheet. The smaller pot is placed inside the larger one and weighed down with a stone.
Sea water is poured into the outer container up to the brim of the inner one. The larger pot is sealed using a plastic sheet and a piece of string so that the plastic sheet sags in the middle (Figure 6). This home-made still is then placed on any heat source such as a stove or wood fire, at low temperature. In a few minutes the sea water in the outer container starts to evaporate. As the plastic sheet prevents the steam from escaping, the droplets condense into the smaller vessel. Residual salt remains in the outer pot. To conserve fuel, a still can be placed on top of a cooking- pot which is used everyday, such as a rice pot. As it boils or simmers, the 'waste' heat is usefully harnessed. Care should be taken to ensure that all pots are stable and out of reach of young children.
Reverse osmosis
Reverse osmosis is a technique which plants use to absorb water from the soil and to transport the water up the stem to all parts of the plant. Dilute and more concentrated solutions are separated by a semi-permeable membrane, which acts like a very fine filter. The semi-permeable membrane allows water molecules to pass, but prevents the movement of salt or other dissolved chemical molecules.
If two saline solutions (or water and a saline solution) are separated only by a semi-permeable membrane, there will be a transfer of water through the membrane to the more concentrated saline solution. The passage of water will continue until a stable condition is reached, with the difference of liquid levels across the semi-permeable membrane being referred to as the osmotic pressure. The osmotic pressure varies with temperature and the concentrations of the two solutions.
By applying pressure (in excess of the osmotic pressure) to the salt-water solution, the process can be reversed, and water molecules from the salt-water solution can be forced through to the other side of the semi-permeable membrane.
Maintenance
Water produced by desalination is low in minerals and usually aggressive towards materials with which it comes into contact, such as materials used for distribution pipes, storage and plumbing. During post-treatment, the water must be stabilized or re-mineralized prior to distribution to reduce its corrosive nature. Stabilization is commonly achieved by adding chemical constituents such as calcium and magnesium carbonate along with pH adjustment or through blending with small volumes of mineral-rich waters.
Costs
Field experiences
Reference manuals, videos, and links
- Safe Drinking-water from Desalination. World Health Organization, 2011.
- Desalination. WEDC Loughborough University.
Acknowledgements
- Guidelines for Drinking-water Quality, fourth edition. World Health Organization (WHO), 2011.
- Smith, Michael, and Shaw, Rod. Desalination. WEDC Loughborough University.