Difference between revisions of "Desalination / Evaporation"
(→Acknowledgements) |
(→Construction, operations and maintenance) |
||
| Line 50: | Line 50: | ||
Salinization of shallow groundwater can occur due to water-logging (e.g. where irrigation is practiced) – this is because of a shallow water table and high evaporation rates. Techniques to reduce salinity include: | Salinization of shallow groundwater can occur due to water-logging (e.g. where irrigation is practiced) – this is because of a shallow water table and high evaporation rates. Techniques to reduce salinity include: | ||
| − | + | * Reducing water table depth. This can be done by reducing groundwater replenishment by lining irrigation canals and using improved irrigation techniques that limit volume of applied water (e.g. drip or sprinkler irrigation), but can also be done by lowering water tables through increasing groundwater discharge through better drainage or planting vegetation with high water consumption rate. | |
| − | + | * Reducing evaporation. Deep tillage can increase soil pores and reduce capillarity, and screens/trees/mulching/high plot perimeters can improve shade and act as windbreaks to reduce evaporation rates. | |
Legislation can impact increasing levels of groundwater salinity – in Mozambique, the government prohibited the drilling of new boreholes in rural areas where salinity was an issue. This combined with rainwater MAR techniques proved to be a good combination at addressing the saline issue. | Legislation can impact increasing levels of groundwater salinity – in Mozambique, the government prohibited the drilling of new boreholes in rural areas where salinity was an issue. This combined with rainwater MAR techniques proved to be a good combination at addressing the saline issue. | ||
Revision as of 01:51, 13 April 2012
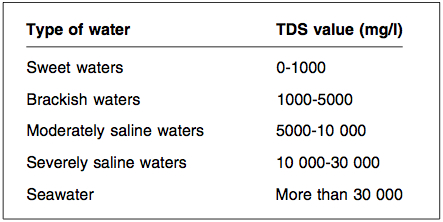
Desalination is used to remove salts from brackish or saline surface water and groundwater in order to render it acceptable for human consumption or other uses. Brackish water is defined as starting at having a Total Dissolved Solids content of 1,000 mg/l, and saline water as having 10,000 mg/l. It is increasingly employed to provide drinking-water because of a growing scarcity of fresh water driven by population growth, overexploitation of water resources and climate change. Desalination facilities exist all over the world, particularly in the eastern Mediterranean region, with use increasing on all continents. Small-scale desalination is used to supply fresh water on ships and to provide additional fresh water in some hot and arid regions.
Desalination by distillation produces water without chemical salts. The method can be expensive because of the capital investment needed and because fuel/charcoal is used to heat the water. However, solar distillation is inexpensive. The volume of water produced is generally low.
Most present applications of desalination are for estuarine water, coastal water and seawater. Desalination may also be applied to brackish inland waters (both surface water and groundwater) and may be used on board vessels. Small-scale desalination units also exist for household and community use and present specific challenges to effective operation and maintenance.
Contents
Suitable Conditions
For use in areas where there are few alternative water sources to the available saline water, therefore before embarking on treatment options for saline water, the first thing to try would be to diversify water sources (e.g. through rainwater harvesting).
Natural waters may be classified approximately according to their Total Dissolved Solids (TDS) values:
Construction, operations and maintenance
Of the desalination methods available, the two main ones are: distillation followed by condensation and reverse osmosis.
Alternative low-cost distillation method
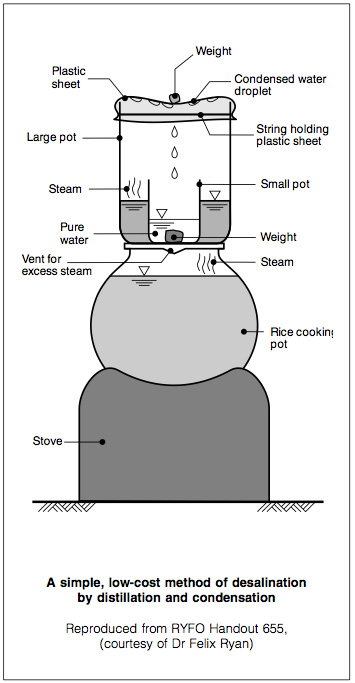
A simple, low-cost method to desalinate sea water by distillation is used in some countries where fuel is available. It requires basic kitchen utensils: two pots, one four times the size of the other, and a plastic sheet. The smaller pot is placed inside the larger one and weighed down with a stone.
Sea water is poured into the outer container up to the brim of the inner one. The larger pot is sealed using a plastic sheet and a piece of string so that the plastic sheet sags in the middle (Figure 6). This home-made still is then placed on any heat source such as a stove or wood fire, at low temperature. In a few minutes the sea water in the outer container starts to evaporate. As the plastic sheet prevents the steam from escaping, the droplets condense into the smaller vessel. Residual salt remains in the outer pot. To conserve fuel, a still can be placed on top of a cooking- pot which is used everyday, such as a rice pot. As it boils or simmers, the 'waste' heat is usefully harnessed. Care should be taken to ensure that all pots are stable and out of reach of young children.
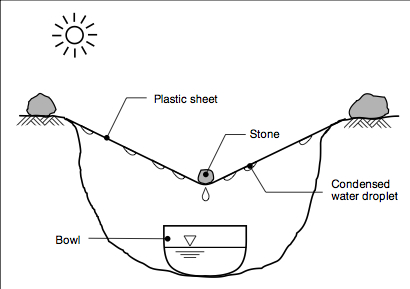
Household solar stills
Household solar stills have not been widely promoted, yet can provide 2.5 – 3 litres per m2 surface area per day. However there is
scope to increase yields – more efficient and expensive stills (Aqua Solaris) have been tried that can increase volume to 40 litres per m2 per day. Water temperatures must be high, while the condensing surface should be as cool as possible – for this reason stills are most efficient in the early evening when water is still warm but temperature of the glass is a lot lower, and stills continue to produce water during the night.
The Watercone is a massproduced innovation that can produce 1.5 litres maximum per cone per day, but tends to be expensive. Solar stills can be constructed using local materials which are cheaper. There are key points to get right:
- Keep water temperature in the solar still as high as possible:
- Use a condensing surface with a low absorption capacity. Glass is most commonly used as it is “wettable” (i.e. water condensing in a film rather than forming droplets which reflect radiation) but should ideally be sufficiently thick to withstand rain, wind and some knocks – 1/8” or 3.2mm is adequate. Plastic should not be used due to the high temperatures.
- Keep water level in the still to between 0.5 – 2.5 cm deep only. This means you'll have less water to heat up and increased efficiency, but not too little that it will dry up.
- Insulate the base and walls of the still. This will retain the heat rather than losing it out the sides and base of the structure. Expanded polystyrene sheets 1” thick is widely available and is good for this purpose. The lining on top of this insulation will retain more heat if it is black.
- Make the solar still waterproof – the easiest way to do this is to use a liner. EPDM or butyl rubber is a good choice as they will not break up or give unpleasant taste/odour to the water.
- Lining the walls inside the solar still with a reflective material (e.g. aluminium foil) may increase reflection of heat energy but has not been tried.
- Avoid vapour leaks. Silicone applied using an application gun works well to seal the glass to the frame.
- Width of glass is normally limited to between 0.65 – 0.9 metres.
- Add 3 times the daily clean water amount each morning to flush the still – water will flow out through the overflow. Failure to do this will result in salts deposited in the still.
- The slope of the glass should be minimal. Water will run off glass even set at 1 degree tilt. As a guide, set the angle so that the distance from water level to glass is in the range of 5 – 7 cm in order to minimize air volume in the still, and to increase efficiency.
- Never allow the still to go dry otherwise it can melt the lining and insulation in the still.
Communal solar stills have been constructed in some locations. Like any other larger communal installations, there have been problems with maintenance. They may work however if they could be owned and managed by people who have a vested interest in the technology (e.g. water vendors).
For volumes of water over 1m3 per day, reverse osmosis (RO) or electrodialysis can be considered. However, for rural areas and most small towns, it will be wise to avoid more complex systems unless you can guarantee technical competence in the design, construction and maintenance of the systems, as well a supply of spare parts and chemicals. If considering this route, it is particularly important is to get a full water analysis done prior to system design, and to have design carried out by RO specialists.
In areas of shallow saline groundwater, Managed Aquifer Recharge (MAR) techniques have been used to dilute this groundwater which can then be re-extracted. Examples are “Tajamar” infiltration ponds in Paraguay and roof water recharge systems in Mozambique.
Salinization of shallow groundwater can occur due to water-logging (e.g. where irrigation is practiced) – this is because of a shallow water table and high evaporation rates. Techniques to reduce salinity include:
- Reducing water table depth. This can be done by reducing groundwater replenishment by lining irrigation canals and using improved irrigation techniques that limit volume of applied water (e.g. drip or sprinkler irrigation), but can also be done by lowering water tables through increasing groundwater discharge through better drainage or planting vegetation with high water consumption rate.
- Reducing evaporation. Deep tillage can increase soil pores and reduce capillarity, and screens/trees/mulching/high plot perimeters can improve shade and act as windbreaks to reduce evaporation rates.
Legislation can impact increasing levels of groundwater salinity – in Mozambique, the government prohibited the drilling of new boreholes in rural areas where salinity was an issue. This combined with rainwater MAR techniques proved to be a good combination at addressing the saline issue.
Reverse osmosis
Reverse osmosis is a technique which plants use to absorb water from the soil and to transport the water up the stem to all parts of the plant. Dilute and more concentrated solutions are separated by a semi-permeable membrane, which acts like a very fine filter. The semi-permeable membrane allows water molecules to pass, but prevents the movement of salt or other dissolved chemical molecules.
If two saline solutions (or water and a saline solution) are separated only by a semi-permeable membrane, there will be a transfer of water through the membrane to the more concentrated saline solution. The passage of water will continue until a stable condition is reached, with the difference of liquid levels across the semi-permeable membrane being referred to as the osmotic pressure. The osmotic pressure varies with temperature and the concentrations of the two solutions.
By applying pressure (in excess of the osmotic pressure) to the salt-water solution, the process can be reversed, and water molecules from the salt-water solution can be forced through to the other side of the semi-permeable membrane.
Maintenance
Water produced by desalination is low in minerals and usually aggressive towards materials with which it comes into contact, such as materials used for distribution pipes, storage and plumbing. During post-treatment, the water must be stabilized or re-mineralized prior to distribution to reduce its corrosive nature. Stabilization is commonly achieved by adding chemical constituents such as calcium and magnesium carbonate along with pH adjustment or through blending with small volumes of mineral-rich waters.
Costs
Solar still costs
- Cost can be reasonably high – between £50-£70 (US$70 - US$100) per m2.
- In Afghanistan, a locally-produced still was priced up at US$65.
- Cost is proportional to output, so there are no economies of scale when scaling up as there are with other treatment methods.
Field experiences
Reference manuals, videos, and links
- Safe Drinking-water from Desalination. World Health Organization, 2011.
- Desalination. WEDC Loughborough University.
- Gabriele Diamanti's "Eliodomestico" solar-powered eco-distiller. Blog.
- New Technology Could Make Desalination More Accessible. New York Times.
Acknowledgements
- CARE Nederland, Desk Study Resilient WASH systems in drought prone areas. October 2010.
- Guidelines for Drinking-water Quality, fourth edition. World Health Organization (WHO), 2011.
- Smith, Michael, and Shaw, Rod. Desalination. WEDC Loughborough University.