Difference between revisions of "Easygel"
(→Costs) |
|||
| Line 1: | Line 1: | ||
| − | [[Image:Example_results_Easygel_test.jpg|thumb|right| | + | [[Image:Example_results_Easygel_test.jpg|thumb|right|200px|Example results Easygel test]] |
| − | __NOTOC__ | + | __NOTOC__ <small-title /> |
Easygel is a replacement product for agar that is more convenient to use and transport. Easygel is a pectin-gel method which comes as a sterile 2-part test unit consisting of a bottle of liquid medium and a petri dish that is pretreated with a special formulation. When the bottle of liquid medium is poured in to the pretreated petri dish, the combination causes the medium to gel. Complete gelling takes around 40 minutes, after which a petri dish is ready to use. There are several formulations of Easygel, ranging from the common Total Count (Standard Plate Count) formulation to the patented Coliscan formulation. <br> | Easygel is a replacement product for agar that is more convenient to use and transport. Easygel is a pectin-gel method which comes as a sterile 2-part test unit consisting of a bottle of liquid medium and a petri dish that is pretreated with a special formulation. When the bottle of liquid medium is poured in to the pretreated petri dish, the combination causes the medium to gel. Complete gelling takes around 40 minutes, after which a petri dish is ready to use. There are several formulations of Easygel, ranging from the common Total Count (Standard Plate Count) formulation to the patented Coliscan formulation. <br> | ||
| Line 9: | Line 9: | ||
Their patented testing methods eliminate the dependence on agar and expensive equipment, and offer the easiest and most effective ways to test water for coliform and E. coli bacteria. | Their patented testing methods eliminate the dependence on agar and expensive equipment, and offer the easiest and most effective ways to test water for coliform and E. coli bacteria. | ||
| − | ==Suitable Conditions== | + | ===Suitable Conditions=== |
Both the Easygel and the MF approaches work very well when used properly. The Easygel® is provided as a liquid medium which contains a gelling agent that causes the medium to solidify when it is poured into a petri dish containing a layer of calcium ions so that it resembles a standard prepoured agar plate. The gelled dish can be streaked or swabbed with test material or a small test sample (generally 1-5 mL) can be added prior to pouring. The MF consists of a liquid medium without a gelling agent which is used to saturate a sterile pad in a small petri dish in conjunction with a micropore membrane filter which has filtered an aqueous test sample, with the sample generally being of a larger volume. Both approaches use very similar formulations and ingredients with the exception of the gelling agent. (For those familiar with the membrane filter approach where the medium is agar based and a solid layer is formed in a dish as the foundation for the membrane filter, Coliscan® can also be prepared in this manner. It is not currently offered in this format other than by custom request.) | Both the Easygel and the MF approaches work very well when used properly. The Easygel® is provided as a liquid medium which contains a gelling agent that causes the medium to solidify when it is poured into a petri dish containing a layer of calcium ions so that it resembles a standard prepoured agar plate. The gelled dish can be streaked or swabbed with test material or a small test sample (generally 1-5 mL) can be added prior to pouring. The MF consists of a liquid medium without a gelling agent which is used to saturate a sterile pad in a small petri dish in conjunction with a micropore membrane filter which has filtered an aqueous test sample, with the sample generally being of a larger volume. Both approaches use very similar formulations and ingredients with the exception of the gelling agent. (For those familiar with the membrane filter approach where the medium is agar based and a solid layer is formed in a dish as the foundation for the membrane filter, Coliscan® can also be prepared in this manner. It is not currently offered in this format other than by custom request.) | ||
The Easygel® is designed for use when the number of target organisms/mL of sample is quite high and therefore the number of colony forming units/plate will be high enough to count with accuracy when a 1-5 mL sample is added directly to the medium. | The Easygel® is designed for use when the number of target organisms/mL of sample is quite high and therefore the number of colony forming units/plate will be high enough to count with accuracy when a 1-5 mL sample is added directly to the medium. | ||
The MF is designed for situations where the test sample has very low numbers of target organisms and it is necessary to validate the absence or very low count of the target organisms, while the Easygel approach is excellent for determining populations of target organisms when they are commonly present in relatively large numbers in the test materials. | The MF is designed for situations where the test sample has very low numbers of target organisms and it is necessary to validate the absence or very low count of the target organisms, while the Easygel approach is excellent for determining populations of target organisms when they are commonly present in relatively large numbers in the test materials. | ||
| − | ==Construction, operations and maintenance== | + | ===Construction, operations and maintenance=== |
[[Image:easygel process.jpg|thumb|right|250px|Pouring the Easygel™ solution into the petri plate. Photo: [http://www.rpcs.org/essre/2002/ESSRE%202002%20Student%20Webs/ESSRE%202002%20%28uv%20+%20mold%29/serialdilutions.htm Rpsc.org]]] | [[Image:easygel process.jpg|thumb|right|250px|Pouring the Easygel™ solution into the petri plate. Photo: [http://www.rpcs.org/essre/2002/ESSRE%202002%20Student%20Webs/ESSRE%202002%20%28uv%20+%20mold%29/serialdilutions.htm Rpsc.org]]] | ||
| − | + | ====Instructions==== | |
Either collect your water sample in a sterile container and transport the water back to the test site, or take a measured water sample directly from the source and place directly into the bottle of Coliscan Easygel. Water samples kept longer than one (1) hour prior to plating, or any Coliscan Easygel bottle that has had a sample placed into it for transport longer than ten (10) minutes, should be kept on ice or in a refrigerator until plated. <br> | Either collect your water sample in a sterile container and transport the water back to the test site, or take a measured water sample directly from the source and place directly into the bottle of Coliscan Easygel. Water samples kept longer than one (1) hour prior to plating, or any Coliscan Easygel bottle that has had a sample placed into it for transport longer than ten (10) minutes, should be kept on ice or in a refrigerator until plated. <br> | ||
# Label the petri dishes with the appropriate sample information. A permanent marker or wax pencil will work. <br> | # Label the petri dishes with the appropriate sample information. A permanent marker or wax pencil will work. <br> | ||
| Line 32: | Line 32: | ||
## Place 5 ml (about 1 teaspoon) of straight bleach onto the surface of the medium of each plate. Allow to sit at least 5 minutes. Place in a watertight bag and discard in trash. <br> | ## Place 5 ml (about 1 teaspoon) of straight bleach onto the surface of the medium of each plate. Allow to sit at least 5 minutes. Place in a watertight bag and discard in trash. <br> | ||
| − | + | ====Interpretation of Results==== | |
This test method utilizes well established, widely accepted criteria for the recognition of coliforms and E. coli and proper application of the method will result in accurate results. Therefore, if you suspect that your water is dangerously contaminated based on the results you get using Coliscan Easygel, you should contact your local health department and ask for their help in performing an official assessment of water. <br> | This test method utilizes well established, widely accepted criteria for the recognition of coliforms and E. coli and proper application of the method will result in accurate results. Therefore, if you suspect that your water is dangerously contaminated based on the results you get using Coliscan Easygel, you should contact your local health department and ask for their help in performing an official assessment of water. <br> | ||
Non-fecal coliforms are widely distributed in nature, being found both as naturally occurring soil organisms, and in the intestines of warm-blooded animals and humans. Fecal coliforms are coliforms found naturally only in the intestines of warm-blooded animals and humans. Fecal coliform contamination is therefore the result of some form of fecal contamination. Sources may be either animal or human. | Non-fecal coliforms are widely distributed in nature, being found both as naturally occurring soil organisms, and in the intestines of warm-blooded animals and humans. Fecal coliforms are coliforms found naturally only in the intestines of warm-blooded animals and humans. Fecal coliform contamination is therefore the result of some form of fecal contamination. Sources may be either animal or human. | ||
| − | ==Costs== | + | ===Costs=== |
Depending on the contents of the kit, and which type of gel is used, the cost of an Easygel kit ranges between $14 and $55. | Depending on the contents of the kit, and which type of gel is used, the cost of an Easygel kit ranges between $14 and $55. | ||
[[Image:Cost_of_Easygel_products.jpg|thumb|none|300px|Cost of Easygel products. Click image to zoom.]] | [[Image:Cost_of_Easygel_products.jpg|thumb|none|300px|Cost of Easygel products. Click image to zoom.]] | ||
| − | ==Field Experiences== | + | ===Field Experiences=== |
| − | * A study of different water testing methods, including Easygel: [http://www. | + | * A study of different water testing methods, including Easygel: [http://www.iwaponline.com/washdev/001/0068/0010068.pdf Comparison and verification of four field-based microbiological tests: H2S test, Easygel®, Colilert®, PetrifilmTM.] |
* An analysis of drinking water contaminant concentrations in the highlands of rural Guatemala, using the Coliscan EasyGel: [http://ascelibrary.org/doi/abs/10.1061/41036%28342%29390 Drinking Water Field Analyses for the Detection and Enumeration of Coliform Bacteria in Rural Guatemalan Highlands] | * An analysis of drinking water contaminant concentrations in the highlands of rural Guatemala, using the Coliscan EasyGel: [http://ascelibrary.org/doi/abs/10.1061/41036%28342%29390 Drinking Water Field Analyses for the Detection and Enumeration of Coliform Bacteria in Rural Guatemalan Highlands] | ||
| − | ==Manuals, videos | + | ===Manuals, videos and links=== |
| − | * PowerPoint presentation: [http:// | + | * PowerPoint presentation: [http://iom.edu/~/media/Files/Activity%20Files/PublicHealth/MicrobialThreats/Sobsey.pdf Current Issues and Approaches to Microbial Testing of Water: Applicability and Use of Current Tests in the Developing World] |
* [http://micrologylabs.mennonite.net/Home/About_Us/History The History of the Development of the Easygel ® and Coliscan ® Methods] | * [http://micrologylabs.mennonite.net/Home/About_Us/History The History of the Development of the Easygel ® and Coliscan ® Methods] | ||
| − | * A PDF based on a PowerPoint presentation: [http://www | + | * A PDF based on a PowerPoint presentation: [http://www.micorps.net/documents/wolfson_ecoli07.pdf E. coli Monitoring – Effective Techniques and Test Kits for Volunteers] |
* Instructions how to use Easygel with images: [http://www.rpcs.org/essre/2002/ESSRE%202002%20Student%20Webs/ESSRE%202002%20%28uv%20+%20mold%29/serialdilutions.htm Serial Dilution Instructions] | * Instructions how to use Easygel with images: [http://www.rpcs.org/essre/2002/ESSRE%202002%20Student%20Webs/ESSRE%202002%20%28uv%20+%20mold%29/serialdilutions.htm Serial Dilution Instructions] | ||
| − | |||
| − | |||
Revision as of 05:21, 3 October 2013
Easygel is a replacement product for agar that is more convenient to use and transport. Easygel is a pectin-gel method which comes as a sterile 2-part test unit consisting of a bottle of liquid medium and a petri dish that is pretreated with a special formulation. When the bottle of liquid medium is poured in to the pretreated petri dish, the combination causes the medium to gel. Complete gelling takes around 40 minutes, after which a petri dish is ready to use. There are several formulations of Easygel, ranging from the common Total Count (Standard Plate Count) formulation to the patented Coliscan formulation.
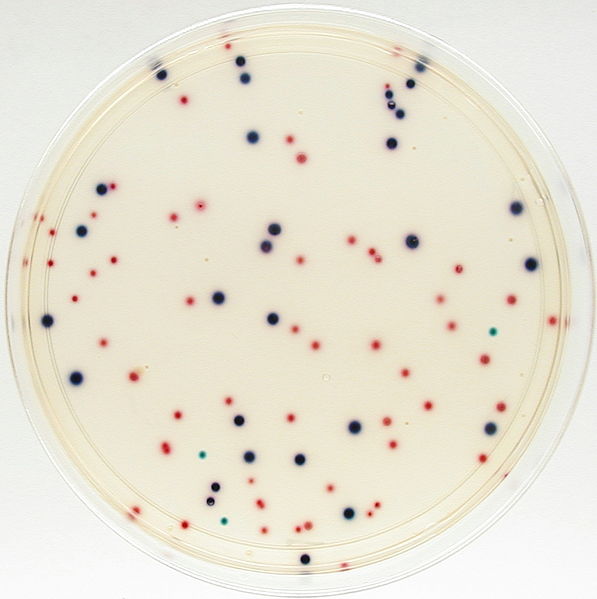
Coliscan media incorporate a patented combination of color-producing chemicals and nutrients that mark coliforms and E. coli in differing colors for easy identification and isolation. This means that a test sample of water or other material may be added to the medium, and coliform bacteria will grow as pink-magenta colonies while E. coli will grow as purple-blue colonies. Other bacterial types will generally grow as non-colored colonies. There are two approaches to the Coliscan method: Coliscan Easygel and Coliscan-MF.
Easygel is produced by Micrology Labaroties, this company specializes in easy-to-use, accurate water testing and microbiology applications.
Their patented testing methods eliminate the dependence on agar and expensive equipment, and offer the easiest and most effective ways to test water for coliform and E. coli bacteria.
Suitable Conditions
Both the Easygel and the MF approaches work very well when used properly. The Easygel® is provided as a liquid medium which contains a gelling agent that causes the medium to solidify when it is poured into a petri dish containing a layer of calcium ions so that it resembles a standard prepoured agar plate. The gelled dish can be streaked or swabbed with test material or a small test sample (generally 1-5 mL) can be added prior to pouring. The MF consists of a liquid medium without a gelling agent which is used to saturate a sterile pad in a small petri dish in conjunction with a micropore membrane filter which has filtered an aqueous test sample, with the sample generally being of a larger volume. Both approaches use very similar formulations and ingredients with the exception of the gelling agent. (For those familiar with the membrane filter approach where the medium is agar based and a solid layer is formed in a dish as the foundation for the membrane filter, Coliscan® can also be prepared in this manner. It is not currently offered in this format other than by custom request.) The Easygel® is designed for use when the number of target organisms/mL of sample is quite high and therefore the number of colony forming units/plate will be high enough to count with accuracy when a 1-5 mL sample is added directly to the medium. The MF is designed for situations where the test sample has very low numbers of target organisms and it is necessary to validate the absence or very low count of the target organisms, while the Easygel approach is excellent for determining populations of target organisms when they are commonly present in relatively large numbers in the test materials.
Construction, operations and maintenance
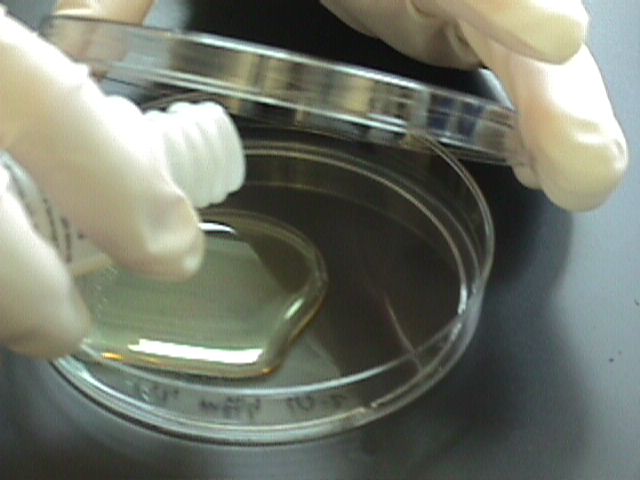
Instructions
Either collect your water sample in a sterile container and transport the water back to the test site, or take a measured water sample directly from the source and place directly into the bottle of Coliscan Easygel. Water samples kept longer than one (1) hour prior to plating, or any Coliscan Easygel bottle that has had a sample placed into it for transport longer than ten (10) minutes, should be kept on ice or in a refrigerator until plated.
- Label the petri dishes with the appropriate sample information. A permanent marker or wax pencil will work.
- Sterilely transfer water from the sample containers into the bottles of Coliscan Easygel (Consult the following table for rough guidelines for inoculum amount). Swirl the bottles to distribute the inoculum and then pour the medium/inoculum mixtures into the correctly labeled petri dishes. Place the lids back on to the petri dishes. Gently swirl the poured dish until the entire dish is covered with liquid (but be careful not to splash over the side or on the lid).
- The dishes may be placed right-side-up directly into a level incubator or warm level spot in the room while still liquid. Solidification will occur in approximately 40 minutes.
- Incubate at 35° C (95° F) for 24 hours, or at room temperature for 48 hours. (See comments on incubation)
- Inspect the dishes
- Count all the purple colonies on the Coliscan dish (disregard any light blue, blue-green or white colonies) , and report the results in terms of E. coli per ml of water. NOTE: To report in terms of E. coli per 100 ml of water, first find the number to multiply by. To do this: first, divide 100 by the number of ml that you used for your sample. Then, multiply the count in your plate by the result obtained from #1. For example, a 3 ml sample, 100 / 3 = 33.3. So, 4 E. coli colonies multiplied by 33.3 will equal 133.2 E. coli per 100 ml of water.
- Count all the pink and purple colonies on the Coliscan dish (disregard any light blue, blue-green or white colonies) and report the results in terms of coliforms per ml of water.
- Count all the purple colonies on the Coliscan dish (disregard any light blue, blue-green or white colonies) , and report the results in terms of E. coli per ml of water. NOTE: To report in terms of E. coli per 100 ml of water, first find the number to multiply by. To do this: first, divide 100 by the number of ml that you used for your sample. Then, multiply the count in your plate by the result obtained from #1. For example, a 3 ml sample, 100 / 3 = 33.3. So, 4 E. coli colonies multiplied by 33.3 will equal 133.2 E. coli per 100 ml of water.
- Do one of the following prior to disposal in normal trash:
- Place dishes and Coliscan bottles in a pressure cooker and cook at 15 lbs. for 15 minutes. This is the best method.
- Place dishes and Coliscan bottles in an ovenproof bag, seal it, and heat in an oven at 300° F for 45 minutes.
- Places dishes and Coliscan bottles in a large pan, cover with water and boil for 45 minutes.
- Place 5 ml (about 1 teaspoon) of straight bleach onto the surface of the medium of each plate. Allow to sit at least 5 minutes. Place in a watertight bag and discard in trash.
- Place dishes and Coliscan bottles in a pressure cooker and cook at 15 lbs. for 15 minutes. This is the best method.
Interpretation of Results
This test method utilizes well established, widely accepted criteria for the recognition of coliforms and E. coli and proper application of the method will result in accurate results. Therefore, if you suspect that your water is dangerously contaminated based on the results you get using Coliscan Easygel, you should contact your local health department and ask for their help in performing an official assessment of water.
Non-fecal coliforms are widely distributed in nature, being found both as naturally occurring soil organisms, and in the intestines of warm-blooded animals and humans. Fecal coliforms are coliforms found naturally only in the intestines of warm-blooded animals and humans. Fecal coliform contamination is therefore the result of some form of fecal contamination. Sources may be either animal or human.
Costs
Depending on the contents of the kit, and which type of gel is used, the cost of an Easygel kit ranges between $14 and $55.
Field Experiences
- A study of different water testing methods, including Easygel: Comparison and verification of four field-based microbiological tests: H2S test, Easygel®, Colilert®, PetrifilmTM.
- An analysis of drinking water contaminant concentrations in the highlands of rural Guatemala, using the Coliscan EasyGel: Drinking Water Field Analyses for the Detection and Enumeration of Coliform Bacteria in Rural Guatemalan Highlands
Manuals, videos and links
- PowerPoint presentation: Current Issues and Approaches to Microbial Testing of Water: Applicability and Use of Current Tests in the Developing World
- The History of the Development of the Easygel ® and Coliscan ® Methods
- A PDF based on a PowerPoint presentation: E. coli Monitoring – Effective Techniques and Test Kits for Volunteers
- Instructions how to use Easygel with images: Serial Dilution Instructions