Difference between revisions of "Chemical disinfection / Free chlorine"
From Akvopedia
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[Image:free chlor icon.png|right| | + | [[Image:free chlor icon.png|right|80px]] |
| − | [[Image:free chlorine.jpg|thumb|right| | + | [[Image:free chlorine.jpg|thumb|right|200px|Free chlorine molecule.]] |
| − | + | <small-title /> | |
Free chlorine does the hard work of killing bacteria and oxidizing contaminants. When you add chlorine to water, you are actually adding free chlorine. When the free chlorine combines with contaminants, it becomes combined chlorine, or chloramines. In water, this form of chlorine has very little sanitizing ability, and no oxidizing ability. Total chlorine is just the sum of both combined chlorine and free chlorine. | Free chlorine does the hard work of killing bacteria and oxidizing contaminants. When you add chlorine to water, you are actually adding free chlorine. When the free chlorine combines with contaminants, it becomes combined chlorine, or chloramines. In water, this form of chlorine has very little sanitizing ability, and no oxidizing ability. Total chlorine is just the sum of both combined chlorine and free chlorine. | ||
| Line 8: | Line 8: | ||
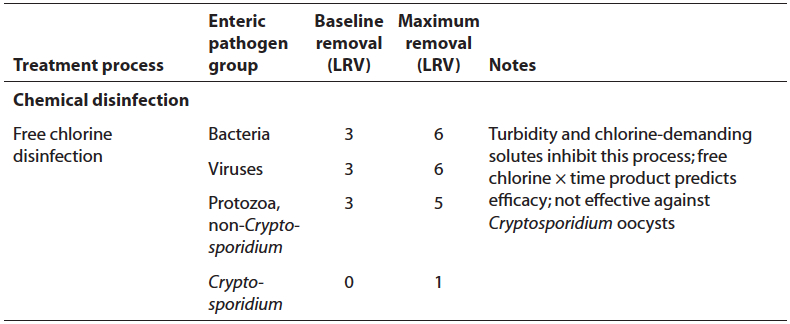
[[File:free chlorine chart.jpg|none|500px]] | [[File:free chlorine chart.jpg|none|500px]] | ||
| − | ==Manuals, videos | + | ===Manuals, videos and links=== |
*[http://www.cdc.gov/safewater/publications_pages/chlorineresidual.pdf Chlorine Residual Testing Fact Sheet.] CDC SWS Project. | *[http://www.cdc.gov/safewater/publications_pages/chlorineresidual.pdf Chlorine Residual Testing Fact Sheet.] CDC SWS Project. | ||
| − | ==Acknowledgements== | + | ===Acknowledgements=== |
* [http://www.uvm.edu/~bwilcke/reiff.pdf Drinking Water Chlorination.] World Chlorine Council, 2008. | * [http://www.uvm.edu/~bwilcke/reiff.pdf Drinking Water Chlorination.] World Chlorine Council, 2008. | ||
Latest revision as of 01:58, 3 October 2013
Free chlorine does the hard work of killing bacteria and oxidizing contaminants. When you add chlorine to water, you are actually adding free chlorine. When the free chlorine combines with contaminants, it becomes combined chlorine, or chloramines. In water, this form of chlorine has very little sanitizing ability, and no oxidizing ability. Total chlorine is just the sum of both combined chlorine and free chlorine.
Chlorine is a versatile and low-cost disinfectant appropriate for any size water system, whether it serves chlorine can also be used for treating water in individual households. Specially-packaged chlorine bleach can disinfect household water for less than US$ 4/year per family.
Manuals, videos and links
- Chlorine Residual Testing Fact Sheet. CDC SWS Project.
Acknowledgements
- Drinking Water Chlorination. World Chlorine Council, 2008.